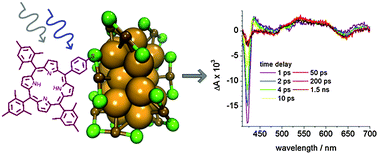
-
Vibrational Coupling Modulation in n- Alkanethiolate Protected Au25(SR)180 Clusters
B. Varnholt, M.J. Guberman-Pfeffer, P. Oulevey, S. Antonello, T. Dainese, J.A. Gascón, T. Bürgi and F. Maran
The Journal of Physical Chemistry C, 120 (44) (2016), p25378-25386


DOI:10.1021/acs.jpcc.6b07592 | unige:94068 | Abstract | Article HTML | Article PDF | Supporting Info
We have studied, both experimentally and theoretically, the Raman vibrational spectra of a series of n-alkanethiolate protected Au25(SCnH2n+1)18 clusters, with n = 2, 3, 4, 5, 6, 8, 10, 12, and 14. The C–H stretching region of the infrared spectra reveals that, while shorter chains are flexible, longer chains are more ordered with a propensity toward extended all-trans conformation. The different behavior of long and short chains is also reflected in the low-frequency Raman spectra of the clusters, which are broadened for the longer chains due to interchain interactions and formation of bundles. The experimental low-frequency modes in the Raman spectra, associated with Au–S stretching vibrations, change drastically and in an apparently unsystematic way as a function of chain length. For example, a band around 320 cm–1 associated with tangential Au–S stretching character shifts up in frequency, then down and then up again as the carbon chain is increased. DFT calculations reveal that this behavior is due to a nonlinear coupling of this mode to torsional and bending modes of the alkyl chain. The frequencies of these modes strongly depend on the chain length and, as a consequence, also their coupling with the Au–S stretching modes, which explains the erratic behavior of this band in the spectra. This behavior is well described by calculations on a mimic cluster model that considers only one staple motif. For the ethanethiolate-protected cluster, the entire cluster was included in the calculation of the Raman spectrum, and this allowed for the first time to compare directly experimental and calculated Raman spectra of the same cluster. Furthermore, our study shows that the entire ligand has to be considered for the calculation of the low frequency vibrations of the Au–S interface, as this spectral region is sensitive to coupling with low-frequency ligand modes.